When I was in graduate school the idea of antimicrobial surfaces was a HOT🔥 research area. Researchers were putting enzymes in coatings, I was making silver nanoparticle modified cellulose nanoparticles (didn’t kill microbes, but cool optical properties), and some non-ionic surfactants showed promising antimicrobial properties. Despite the road to how these materials were developed the need for them back was thought to be high before we ever thought about a pandemic fundamentally changing our lives in 2020.
Hospitals, airplanes, and public transportation have long been considered breeding grounds for transmission of microbes and viruses. If you have ever ridden a crowded subway train in New York City, Washington D.C., or Chicago then you know what I mean. The idea that a surface such as a hospital wall or a seat on a subway car could be inherently antimicrobial has been the lofty goal scientist and engineers wanted to achieve. The thought of staying in a hospital for long term care is a bit scary considering how many sick people are there. Covid-19 exposed our fragility as a society and civilization.
Last year I wrote about PPG and Corning coming out with a coating that could kill microbes and viruses including Covid-19. The technology primarily functioned on maintaining copper ions in a cermaic carrier (Corning), which could be incorporated into a coating (PPG).
Move over PPG and Corning because Kraton has entered the chat.
In the News
Multiple chemistry news organizations have reported that the EPA has granted emergency approval to Kraton’s new polymer BiaXam to help combat Covid-19. Anthony Locicero reported for Coatings World that the polymer was approved by the EPA according section 18 of FIFRA due to submissions by Georgia, Utah, and Minnesota. Delta Airlines will be the first to deploy BiaXam in specific applications.
Locicero also provided quotes on the new polymer:
"We believe that BiaXam is unique due to both its efficacy, durable and residual properties that distinguish it from other technologies that require a more frequent application or treatment," said Kevin M. Fogarty, Kraton's president, and CEO. "We are not aware of other available technologies that provide the long-lasting, durable protection that BiaXam can offer.
"Moreover, while other antimicrobial products are based upon a chemical as the active ingredient, BiaXam is a polymer, and the antimicrobial properties are an inherent feature of the polymer design," he added.
"This new technology offers an exciting new layer of protection that will help us maintain and enhance the effectiveness and efficiency of our protocols and build customer confidence both during the pandemic and beyond," said Mike Medeiros, VP of Global Cleanliness at Delta.
I guess Kevin Fogarty didn’t see that PPG and Corning applied for EPA approval back in November 2020. The BiaXam polymer is reported to be a sulfonated polymer and was developed to be part of Kraton’s sulfonated polymer product line. What is a sulfonated polymer and how does this actually function as an antimicrobial surface? Today, I’ll attempt to provide some clarity around how this all works.
Sulfonated Polymers
A Covid-19 killing material sounds like scientists got together and developed a special weapon to slay the Covid-19 dragon. In reality we have had these polymers available to us for quite a long time. Chemists and chemical engineers have been using sulfonated polymers for a long time, specifically in applications related to heterogeneous catalysis and ion exchange resins where these sulfonated polymers are often small micro porous beads that contain sulfonate/sulfonic acid groups.
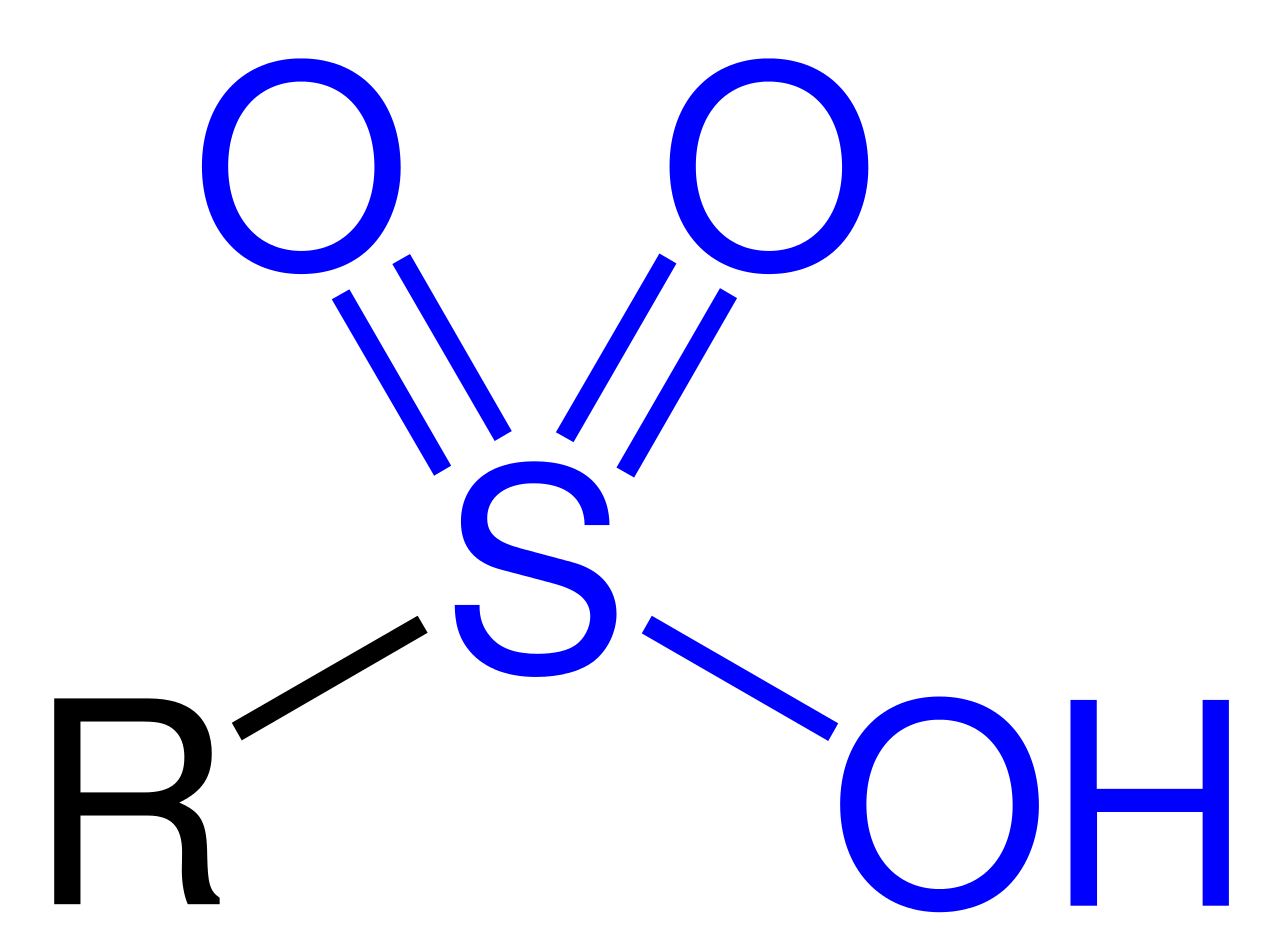
What is a sulfonic acid or sulfonate group and are they different or similar? I have an image of a sulfonic acid group above. I’m not attempting to give an organic chemistry lesson here. When a chemist shows you a structure they are doing what a zoologist might do when you ask them, “what is an elephant?” Sulfonic acids for this instance are going to be attached to our polymer and is represented by the letter R. Many sulfonic acids attached to a small polymer bead can yield similar properties to a liquid sulfonic acids, but sulfonic acids immobilized on a polymeric bead have the benefit of being a solid and less hazardous than a liquid acid.
A sulfonate is essentially a sulfonic acid without the proton (hydrogen atom) and could be balanced with a positive ion such as a sodium (Na). In the picture above I am showing a sulfonate without a counter charge (thanks wikipedia, also I don’t have ChemDraw) A sulfonated polymer would be used to exchange ions in a medium such as water and this is how some water softening technologies might work. A sulfonic acid is a strong acid where the proton is present and exchanging the proton for a sodium is how one would generate sodium sulfonate. Polymers containing sulfonic acid groups like Amberlyst resin beads (DuPont) can be useful catalysts for things requiring acid catalysts.
Rohm and Haas as far as I know were one of the first to develop these sulfonic acid or sulfonate resins under the trade names of Amberlyst or Amber respectively. Dow would eventually buy Rohm and Haas and then would merge with DuPont to form DowDupont and then once they split the business would reside with DuPont. I’ve seen Jim Bohling give a talk more than once and he used to work in this area for Rohm and Haas (I think he works for Dow still) and there is a well defined synthetic pathway for making these types of resins with some steps that are quite dangerous. But why would you ever want your acids on polymer, is there something wrong with regular liquid acids?
Let’s say you want to do a classic undergraduate organic lab and esterify some acetic acid with isoamyl alcohol. This is known as a “Fischer esterification” and it is classically done with a strong acid catalst like hydrochloric acid or sulfuric acid. Once the reaction is complete this acid needs to be taken care of (neutralized) and it is typically the first introduction of a “work-up” or removing unwanted chemicals from your reaction including the acid catalyst. I’ve seen this lab performed using Amberlyst resins and having some students filter out the catalyst and thus their workup becomes as simple as removing one of the excess reactants and not needing to neutralize the catalyst in the reaction mixture. Neutalizations of acid can create a lot of waste water, which is expensive to process.
There are plenty of applications for heterogeneous catalysis that I will not get into here, but my point of going down this short history of sulfonated polymers is to show that knowledge of how to make these polymers exists. It’s not unusual to know the science behind how many things work for decades, but it takes investors and commercially minded people decades to get comfortable in deploying these technologies. Or, a world changing pandemic accelerates risk tolerance and suddenly not deploying these products now seems like the biggest risk of all.
On sulfonic modified polymers having antimicrobial properties a recent paper by Peddinti and coworkers was published in Material Horizons in 2019. The collaboration featured a scientist with Proctor and Gamble and explored polymers similar to Kraton’s BiaXam. Peddinti and coworkers wrote:
Here, we demonstrate that charged multiblock polymers wherein the midblock is selectively sulfonated, and therefore hydrophilic and water-swellable, inherently provide self-sterilizing surfaces that rapidly act (killing more than 99.9999% in just 5 min) against a wide range of Gram-positive and -negative bacteria, three of which are antibiotic-resistant. This surprising response, which depends on the degree of midblock sulfonation, is attributed to a dramatic reduction in surface pH level that is remarkably effective against microbes with a typically anionic outer membrane. These polymers, suitable for use in biomedical applications, smart textiles, separation membranes, commodity fixtures, and food packaging, are equally effective against infectious virus strains.
Peddinti and coworkers were not the first to report these results though.
Kraton Enters:
The only mention of antimicrobial polymers with Kraton in the public patent literature that I can find was in US 20070021569A1. I am not looking to do a full on patent map here so my searching was cursory at best, but my point is that the scientists at Kraton have been aware of this area for some time. From US 20070021569A1 paragraph 160:
The sulfonated block copolymers according to the present invention can be used in a variety of applications and end uses…Articles fabricated from these types of membranes could have antibacterial and/or antiviral and/or antimicrobial properties as reported in U.S. Pat. Nos. 6,537,538, 6,239,182, 6,028,115, 6,932,619 and 5,925,621 where it is noted that polystyrene sulfonates act as inhibitory agents against HIV (human immunodeficiency virus) and HSV (herpes simplex virus).
I’ll refer to this patent as the Willis patent (the lead inventor) and it is currently granted and active according to Google Patents. What I find interesting is that despite knowledge of potential antimicrobial/antiviral applications it has taken Kraton this long to come out with a product specifically aimed at controlling or mitigating microbes or viruses on surfaces. It should be noted that the emergency approval is for mitigating the SARS-CoV-2 virus only. The Willis patent was filed in 2005, but we would not have a Covid-19 global pandemic until 15 years later, but in theory the coating should work against some significant amount of viruses if the patent literature is reporting HIV and HSV for similar materials.
I do not have complete information here, but I will speculate that it took Kraton this long to get a product to market because of the following reasons:
Antiviral and antibacterial materials are not core to Kraton’s business
The physical properties of materials that possessed effective pathogenic inhibition up until a few years ago were not very good or swelled significantly in the presence of water
The risk/reward calculation that Kraton’s product team probably conducted was that their business was just fine without needing to go the route of developing an antibacterial/microbial/viral material. Why take on the additional monstrous liability in the event something went wrong with their new products?
As for physical properties of polymers, its often an issue of solving for two problems that are in opposition to each other. Kraton’s polymer might have killed viruses, but it might not have possessed any properties that would make it useful in the real world. Solving to get both properties in one material might be a pending patent at the USPTO right now.
Note: I am not a lawyer and the following is not a legal opinion, but rather speculative entertainment
The fact that the Willis patent even discloses antimicrobial properties to me is somewhat strange. Much of the patent’s specification is focused on fuel cell applications and fluid transport—not antimicrobial applications. Further, to bring up antimicrobial applications in the specification could also open them to more prior art so I’m guessing that Kraton wanted to make it more difficult for anyone else that followed in their footsteps to patent antimicrobial materials based on their products. I was told that this strategy is referred to as “poisoning the well.” The paragraph I quoted above is an example of prior art against any future IP around using a Kraton polymer as an antimicrobial agent.
The Risk Reward Calculation Has Changed
I suspect that this was a calculated move on the part of Kraton to enter a new market with an existing and slightly modified technology. In the words of Winston Churchill, “Never waste a good crisis.” People will have more suspicion when it comes to traveling in any sort of shared or public transportation environment where there could be active pathogens. Think about traveling on a subway car or in an airplane and many of the surfaces you touch are likely a polymer of some sort. The question that Kraton is hoping you are asking yourself is, “Do I trust that this surface was disinfected properly before I touched it?”
In a post-Corona world people may generally be more fearful of indoor common areas or shared spaces and thus risk could be everywhere. Kraton is asking, “why not sell insurance against these risks in the form of pathogen killing polymeric materials?”
Kraton is betting that we will want our surfaces to kill pathogens. I can’t wait to see these things deployed in the real world. I could be wrong in my assumptions here too, but I just want to reiterate that this technology of polymers containing sulfonates/sulfonic acids has been known for a long time. The antimicrobial properties have been known for over 15 years. It’s good to see these old technologies finally making it to the commercial markets.
An interesting article I read recently while researching how to write this post came from The Spokane Review written by Elizabeth Mayhew who was covering the PPG/Corning story:
“I don’t think antimicrobial paints are the key to preventing the spread of disease,” says Erica Marie Hartmann, assistant professor of civil and environmental engineering at Northwestern University. “Specifically, in the context of COVID-19, evidence points to viruses in the air being the main way the disease spreads. Paints won’t help with that.”
For the most part I agree, making walls antimicrobial probably will not save us, but making air filters, catheters, or high touch surfaces with these new virus and bacteria killing materials could make a significant impact. I choose to believe that good polymer science can still make our lives better, but then again I might be slightly biased.
Talk to you Friday,





Definitely a hot area. One aspect that has never been clear to me is what the actual public health risks of microbe-contaminated *surfaces* are (outside of health care settings). Is there an actual benefit to having things like doorknobs, etc. be made anti-microbial? Is there a resistance trade-off, like there is in antibiotic drugs?
And by extension -- can we assume that anti-bacterial surface treatments will necessarily be effectively anti-viral? (My reading on this seems to suggest the answer is "not always")
Thanks, Tony, nice job. Bringing a technology to a product is really a long journey, the reason why antimicrobial/antivirus products are highly risky might be due to the headache dealing with FDA or EPA, did not work with them directly before, but got involved in a project requiring FDA's approval, it takes years.